
Of the over 110 elements currently known, only elements up to number 92, uranium, occur natually. The remaining elements in the periodic table are all Synthetic. Synthetic elements are made from lab scientists around the world, in other words, synthetic elements are man-made. Synthetic elements required technology and human ingenuity on the grandest scale.
Naturally occurring elements were forged in violent unions of matter and energy in deep space. With the expection of hydrogen all netural elements found on earth were manufactured in the interiors of stars that exploded long before our solar system came into being. You can find naturally occurring elements and synthetic in periodic table.
 We found interesting article that announced about new super-heavy element created. Here is the article!
We found interesting article that announced about new super-heavy element created. Here is the article!
New superheavy elements created
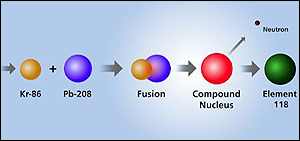
Two new "superheavy" elements have been made by bombarding lead atoms with energy-packed krypton atoms at the rate of two trillion per second.
After 11 days, the scientists working at the Lawrence Berkeley National Laboratory, US, had produced just three atoms of element 118. These each contained 118 protons and 175 neutrons in their nucleii.
The new atoms decayed almost instantly to element 116, which itself was short-lived. But, for that brief moment, they were the only three atoms of these elements ever to have existed on Earth.
Ken Gregorich, the nuclear chemist who led the discovery team, said: "Our unexpected success in producing these superheavy elements opens up a whole world of possibilities using similar reactions: new elements and isotopes."
US Secretary of Energy, Bill Richardson, commented: "This stunning discovery opens the door to further insights into the structure of the atomic nucleus."
Unstable combination
Atoms consist of a central nucleus surrounded by a cloud of electrons. The nucleus consists of protons and neutrons.
But not all combinations of neutrons and protons are stable. In nature, no element heavier than uranium, with 92 protons and 146 neutrons, can normally be found.
Scientists can make heavier ones by colliding two large nuclei together and hoping that they will form a new, heavier nucleus for a short time.
One of the most significant aspects of the new elements is that their decay sequence is consistent with theories that predict an "island of stability" for atoms containing approximately 114 protons and 184 neutrons.
"We jumped over a sea of instability on to an island of stability that theories have been predicting since the 1970s," said nuclear physicist Victor Ninov. He is the first author of a paper on the discovery submitted to Physical Review Letters journal.
Atomic structure
Synthetic elements are often short-lived, but provide scientists with valuable insights into the structure of atomic nuclei. They also offer opportunities to study the chemical properties of the elements heavier than uranium.
I-Yang Lee, scientific director of the atom smasher at Lawrence Berkeley National Laboratory, said "From the discovery of these two new superheavy elements, it is now clear that the island of stability can be reached.
"Additionally, similar reactions can be used to produce other elements and isotopes, providing a rich new region for the study of nuclear properties."
Element 118 takes less than a thousandth of a second to decay by emitting an alpha particle. This leaves behind an isotope of element 116 which contains 116 protons and 173 neutrons.
This daughter is also radioactive, alpha-decaying to an isotope of element 114. The chain of successive alpha decays continues until at least element 106.
![]() Bibliography: BBC News-Sci/Tech
Bibliography: BBC News-Sci/Tech
![]() What trends are found in the periodic table?
What trends are found in the periodic table?![]() How How can atoms be counted or measured?
How How can atoms be counted or measured?
