Sometimes a reaction is desirable, especially if the new substances have special uses because of their properties. For example, aspirin can be made from acetic acid, and carbon dioxide, and phenol. There are 5 types of chemical reaction: synthesis, decomposition, single-displacement, double-displacement, combustion. I will explain each type by step.
1. Synthesis
- Two or more simple substances combine to form a more complex substance. Two or more reactants yielding one product are another way to identify a synthesis reaction.
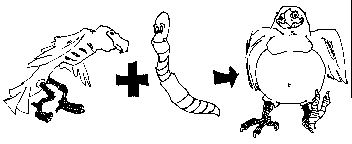
In the cartoon, the skinny bird (reactant) and the worm (reactant) combine to make one product, a fat bird.
2. Decomposition
A. It is a chemical reaction in which a single compound is broken down to produce two or more simpler substances.
B. Synthesis and decomposition is opposite.
C. Reaction produces product and product.
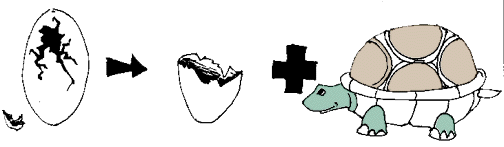
In this cartoon the egg (the reactant), which contained the turtle at one time, now has opened and the turtle (product) and egg shell (product) are now two separate substances.
3. Single displacement
- It is a chemical reaction in which one element replaces another element in a compound.

Before the reaction begins, atoms of zinc are in the elemental form, and atoms of hydrogen are combined in a compound with chlorine. After the reaction, the atoms of hydrogen are in the elemental form, and atoms of zinc are combined in a compound with chlorine. In effect, the zinc atoms and hydrogen atoms switch places.
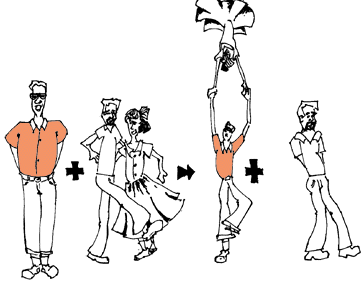
Notice, the guy in the orange shirt steals the date of the other guy. So, a part of one of the reactants trades places and is in a different place among the products.
4. Double displacement
- It is a chemical reaction in which two elements in different compounds exchange places.

5. Combustion
- It is a exothermic reaction that usually involves oxygen to form the oxides of the elements in a reactant.
![]() What is a chemical reaction?
What is a chemical reaction?
![]() How are reaction written?
How are reaction written?
