I will explain from now about electric current. Let me take one example to explain. Strip of zinc metal standing in an aqueous solution of copper (ll) sulfate is the one. Imagine how it will look like.
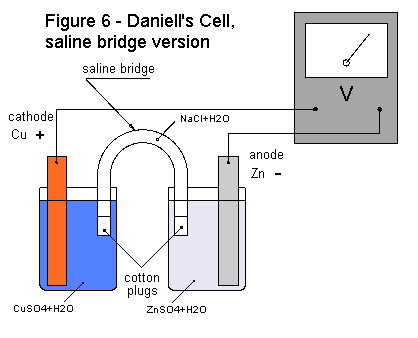
This preparation is called Daniell¡¯s cell porous vase version. In the Daniell's Cell, the copper strip attracts electrons from the zinc strip. These electrons pass through the wires of our external circuit. As the copper electrode receives electrons, free positive ions in the solution arrive to equalize the charges. Positive copper ions (Cu++) are attracted to the charged copper electrode where they receive two electrons and become neutral and deposit on the electrode in metallic form. The positive zinc ions (Zn++) move to the porous vase. For each copper atom that is deposited on the copper electrode, a zinc atom goes into solution, giving up two electrons to the zinc electrode.
The reactions at the electrodes can be represented by this formula:
Cu++ + 2e- ====> Cu
These reactions result in the dissolution of zinc atoms in their ionic form, which corresponds to the deposition of copper ions in their metallic form:
The electrons made available by the zinc atoms pass through the lamp filament, produce light through the joule effect and eventually reach the copper electrode. These electrons account for the current that is produced by the battery and is used by the lamp. If we didn't have the porous vase, the Cu++ ions would go directly to the zinc electrode and pick up free electrons, thereby bypassing the external circuit and stopping the current flow through the wires and lamp. The battery would no longer work. Because the copper electrode attracts electrons from the external circuit, it is considered the positive pole of the battery.
![]() How are electron transfers described?
How are electron transfers described?
![]() How do batteries work?
How do batteries work?
